Non-surgical, self-administered treatment for HPV-induced neoplasia/cancer
Frantz Viral Therapeutics (FVT) is a joint venture between Georgetown University Medical Center and Frantz Medical Group. It is focused on the treatment of the human papillomavirus (HPV)-induced conditions, including pre-cancers (intraepithelial neoplasia) and cancers located in the cervix, vulva, anus, and oropharynx.
The proprietary, patented treatment includes administering therapeutic-effective amounts of artemisinin-related compounds and combinations with other agents. These compounds are administered to patients through various methods including as a vaginal insert, anal suppository or ointment. These treatments are self-administered. The artemisinin-related compound, artesunate, has treated millions of malaria patients worldwide, and proven to be safe. FVT continues to develop other proprietary related compounds.
Background Image: Human Papillomavirus (HPV)
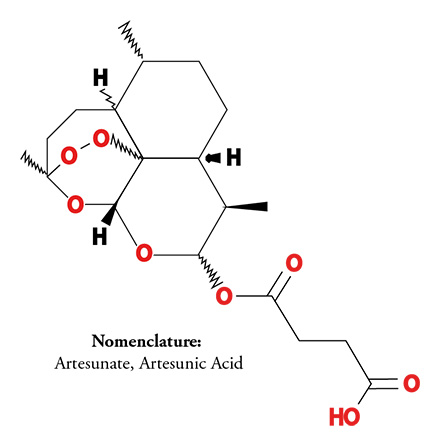
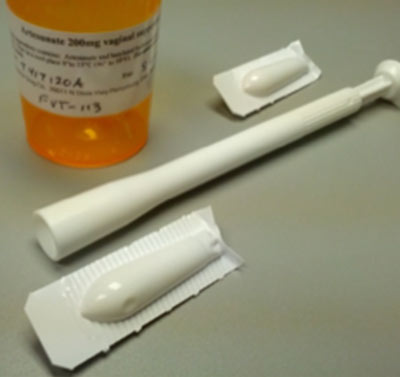
The human papillomavirus (HPV) is thought to be responsible for more than 90% of cervical and anal cancer, and about 70% of vulvar cancers. Worldwide about 300,000 women die annually due to cervical cancer, primarily because of the lack of comprehensive screening programs in low and middle-income countries (LMIC). Additionally, the incidence of other HPV-associated cancers, such as anal, vulvar, penile, and vaginal cancers, are on the rise. The artesunate treatment addresses the pre-malignant stage of these diseases, referred to as neoplasia/dysplasia and manifested as lesions. Most treatment options currently available are surgical or ablative. The artesunate therapy is self-administered, thus addressing treatment resource limitations in LMIC. In the U.S. alone, approximately 500,000 women undergo cervical tissue excision or ablation of these lesions annually. The artesunate treatment for cervical HSIL provides a non-surgical, safe therapy within the standard of care window still allowing for excision/ablation procedures in the event that the artesunate treatment is not effective. While anal and vulvar cancers are less common, the incidence of both has increased significantly in the past decade. Patients diagnosed with these cancers experience significant health and quality of life issues due to morbidities associated with surgical treatments. It is our belief that the results of the ongoing clinical trials will offer non-surgical treatment options for these three precursor lesions.
Dr. Richard Schlegel and his team at Georgetown University Medical Center are the inventors of this novel treatment. Dr. Schlegel has been an expert in the HPV field for decades and his accomplishments include the development of the HPV vaccine. Building upon Dr. Schlegel’s work, Frantz Viral Therapeutics with the collaboration of multiple experts in the field of HPV disease treatments continued the translational work with 3 Phase I/IIA clinical trials using artesunate in different formulations and doses for the treatment of cervical, anal, and vulvar pre-malignancies, respectively. Dr. Cornelia Trimble, Director of Johns Hopkins Center for Cervical Dysplasia, was the Global Principal Investigator of both Phase I/IIA clinical trials of artesunate treatments for cervical intraepithelial neoplasia and vulvar high grade squamous intraepithelial lesions. The Cleveland Clinic was the primary site for the Phase I/IIA vulvar trial under the direction of Dr. Chad Michener as site Principal Investigator. Dr. Sandy Fang was the Principal Investigator of the Phase I/IIA anal high grade squamous intraepithelial lesion clinical trial completed at Johns Hopkins and University of Wisconsin Madison.
Due to excellent safety and efficacy results with the phase I/IIA studies, Phase IIB studies of artesunate for cervical and vulvar HSIL have been initiated with the support of leaders in their respective fields. Cleveland clinic: Dr. Chad Michener; M.D. Anderson: Drs. Andrea Milbourne and Kathleen Schmeler; University of Michigan: Dr. Diane Harper.
Dr. Joel Palefsky at UCSF is the Global Principal Investigator for the anal Phase IIB clinical trial of artesunate suppositories for HIV-negative patients, and Drs. Stephen Goldstone, Joseph Terlizzi, and Gary Bucher, are Site Principal Investigators in New York and Chicago. The AIDS Malignancy Consortium plans to conduct a similar artesunate trial for HIV-positive patients.
Management Team
Chairman/CEO, Frantz Viral Therapeutics
Former Chair, Cleveland Clinic Lerner Research Institute Leadership Council
Former Co-Chair, Georgetown University Medical Center Steering Committee
Director, Frantz Viral Therapeutics Clinical Research Programs
Former Johns Hopkins Clinical Research Manager
Scientific Advisors
Chair, Frantz Viral Therapeutics Clinical Advisory Board
President, Preventive Oncology International Inc.
Former Chairman, Department of Obstetrics and Gynecology, Cleveland Clinic
Professor of Surgery, Cleveland Clinic Lerner College of Medicine
Cleveland, Ohio
Marc S. Penn, MD, PhD
Professor of Integrative Medical Sciences and Professor of Internal Medicine
Northeast Medical University
Rootstown, Ohio
Professor and Hunter Endowed Chair, Department of Pathology
Director, Cell Reprogramming Center
Georgetown University School of Medicine
Washington, DC
Cornelia L. Trimble, MD
Professor, Departments of Gynecology & Obstetrics, Pathology and Oncology
Director, Center for Cervical Dysplasia
Johns Hopkins University School of Medicine
Baltimore, Maryland
