Non-surgical, self-administered treatment for HPV-induced neoplasia/cancer
Frantz Viral Therapeutics (FVT) is a joint venture between Georgetown University Medical Center and Frantz Medical Group. It is focused on the treatment of the human papillomavirus (HPV)-induced conditions, including pre-cancers (intraepithelial neoplasia) and cancers located in the cervix, vulva, anus, and oropharynx.
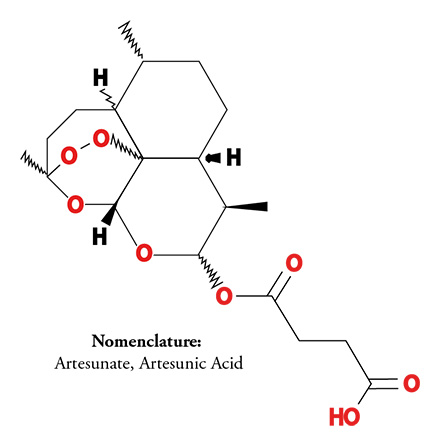
The proprietary, patented treatment includes administering therapeutic-effective amounts of artemisinin-related compounds and combinations with other agents. These compounds are administered to patients through various methods including as a vaginal insert, anal suppository or ointment. These treatments are self-administered. The artemisinin-related compound, artesunate, has treated millions of malaria patients worldwide, and proven to be safe. FVT continues to develop other proprietary related compounds.
Background Image: Human Papillomavirus (HPV)
Dr. Richard Schlegel and his team at Georgetown University Medical Center are the inventors of this novel treatment. Dr. Schlegel has been an expert in the HPV field for decades and his accomplishments include the development of the HPV vaccine. Dr. Cornelia Trimble is the Principal Investigator of both Phase I clinical trials of artesunate treatments for cervical intraepithelial neoplasia (CIN2/3) and vulvar intraepithelial neoplasia (VIN2/3). She has been a leader in the field of HPV research and treatments for nearly 20 years. Dr. Sandy Fang was the Principal Investigator of the Phase I anal intraepithelial neoplasia (anal HSIL) clinical trial completed at Johns Hopkins and University of Wisconsin Madison, and the upcoming multi-institution phase IIB trial of artesunate suppositories for anal HSIL. Dr. Chad Michener is the Site Principal Investigator of the Phase IIB CIN2/3 and Phase I VIN2/3 clinical trials at the Cleveland Clinic. Drs. Andrea Milbourne and Kathleen Schmeler are co-Principal Investigators of the Phase IIB CIN2/3 clinical trial at MD Anderson/LBJ Hospital.
Two phase I studies of artesunate for cervical and anal HSIL have been completed. Both of these trials have demonstrated safety and efficacy. The Phase I trial of artesunate ointment for vulvar HSIL is now closed to enrollment. The Phase IIB cervical clinical trial is currently enrolling. Active clinical trial sites are: MD Anderson/LBJ Hospital, and Cleveland Clinic. Additional sites will be added in the near future. The phase IIB study of artesunate suppositories for anal HSIL will open to enrollment soon at Oregon Health and Science University (Sandy Fang, PI).
![]()
The human papillomavirus (HPV) is thought to be responsible for more than 90% of cervical and anal cancer, and about 70% of vulvar cancers. Worldwide about 300,000 women die annually due to cervical cancer, primarily as a result of the lack of comprehensive screening programs in low and middle-income countries. The artesunate therapy is self-administered, thus addressing treatment resource limitations. In the U.S. alone, about 500,000 women undergo cervical tissue excision or ablation yearly. The artesunate treatment provides a non-surgical, safe therapy within the standard of care window still allowing for excision/ablation procedures, if necessary, at the end of the window.
While anal and vulvar cancers are less common, the incidence of both has increased significantly in the past decade. Patients diagnosed with these cancers experience significant health and quality of life issues due to morbidities associated with surgical treatments. It is our hope that the results of the ongoing clinical trials will offer non-surgical treatment options for these two cancers by targeting the precursor lesions, anal HSIL and vulvar HSIL, respectively.
![]()
Management Team
Chairman/CEO, Frantz Viral Therapeutics
Director, Research Programs
Scientific Advisors
President, Preventive Oncology International Inc.
Former Chairman, Department of Obstetrics and Gynecology, Cleveland Clinic
Professor of Surgery, Cleveland Clinic Lerner College of Medicine
Cleveland, Ohio
Marc S. Penn, MD, PhD
Professor of Integrative Medical Sciences and Professor of Internal Medicine
Northeast Medical University
Rootstown, Ohio
Professor and Hunter Endowed Chair, Department of Pathology
Director, Cell Reprogramming Center
Georgetown University School of Medicine
Washington, DC
Cornelia L. Trimble, MD
Professor, Departments of Gynecology & Obstetrics, Pathology and Oncology
Director, Center for Cervical Dysplasia
Johns Hopkins University School of Medicine
Baltimore, Maryland
